
Born with a bad seed
Lp(a) is similar to LDL cholesterol, sometimes called 'bad cholesterol’,but is more sticky, increasing risk of blockages and blood clots in arteries.Common LDL lowering drugs such as statins don't have the same lowering effect on Lp(a). Being largely genetic,Lp(a) is also difficult to control through diet, exercise and other lifestyle changes. Although Lp(a) was discovered nearly 60 years ago there still aren't any widely accessible treatments available to lower levels and reduce cardiovascular risk. The researcher says that this drug is a gamechanger in more ways than one. It can be delivered in an oral table. Lp(a) is essentially a silent killer with no available treatment, this drug changes that. This drug may also have potential to be used in the treatment of other vascular and valve disease.
A silver lining
A new drug offers a breakthrough world first treatment for Lipoprotein(a), a largely genetic form of cholesterol that increases the risk of heart attack and stroke. High levels of Lipoprotein(a), known as Lp(a), impact one in five people globally with no approved treatment currently on the market. The trial demonstrated the success of Muvalaplin - the first oral drug ever developed to target Lp(a)- effectively lowering levels by up to 65%. It works by disrupting the ability for Lp(a) to form in the body. Monash University researchers published their research results in the journal JAMA1.
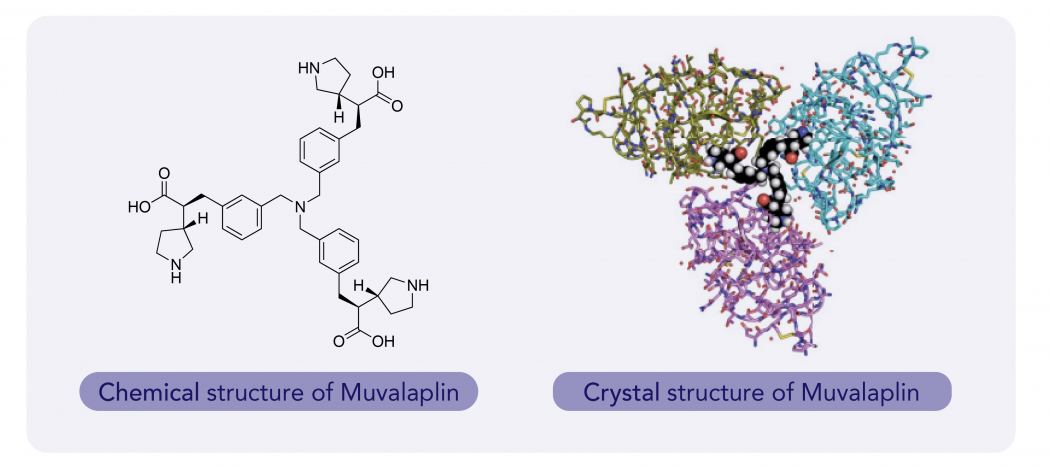
Graceful triple helix
Crystal structure analysis of the interactions between Muvalaplin and KIV8 shows that the trimeric molecule is capable of binding to three KIV8 domains simultaneously, which suggests that multi-KIV domain interactions are possible in apo(a) . Muvalaplin-HCl salt binds to KIV8 selectively with a potency of 22 nM and inhibits the formation of Lp(a) particles in vitro with an IC50 value of 0.09 nM (n = 9). In the Lp(a) transgenic mouse model, the plasma levels of LY3473329-HCl salt increased in a dose-dependent manner, and treatment with Muvalaplin-HCl reduced the levels of Lp(a) with an absolute ED50 of 3 mg kg−1, with a maximum Lp(a) reduction of 92% after five days of BID oral dosing. In cynomolgus monkeys, Muvalaplin reduced median Lp(a) levels in a dose-dependent manner by up to 71% in the 100 mg kg−1 once daily (QD) cohort, compared with baseline. Multivalency, which allowed the engagement of multiple apo(a) KIV domains, considerably increased the potency and efficacy of inhibitors of Lp(a) formation in both in vitro and in vivo models of Lp(a) reduction, as compared with monovalency.2
Latest progress
The newest Eli Lilly and Company recently announced positive results in a Phase 2 clinical trial for Muvalaplin showed that the trial met its primary endpoint, and Muvalaplin was able to significantly reduce Lp (a) levels in adult patients, showing significant improvement in the percentage change in Lp (a) levels from baseline to week 12. At the 12-week primary endpoint, Muvalaplin (10 mg, 60 mg and 240 mg) showed significant reductions in Lp(a) levels compared to placebo. The placebo-adjusted reductions were up to 85.8% using an intact Lp(a) assay and up to 70.0% using an apo(a) assay. Specifically, the reductions were 47.6% (10 mg), 81.7% (60 mg) and 85.8% (240 mg) with the intact Lp(a) assay, and 40.4% (10 mg), 70.0% (60 mg) and 68.9% (240 mg) with the apo(a) assay.
LinkChem Lp(a) buildingblock series
.png)
Reference:
1. Nicholls SJ, Nissen SE, Fleming C, Urva S, Suico J, Berg PH, et al. Muvalaplin, an oral small molecule inhibitor of lipoprotein(a) formation: a randomized clinical trial. JAMA. 2023;330(11):1042–53
2. Nuria D, Carlos P, Ana ME, Gema S, Julian P, Laura FM , et al. Discovery of potent small-molecule inhibitors of lipoprotein(a) formation. Nature. 2024;629:945–950
This article is published by LinkChem Technology Co., Ltd. Welcome to scan the QR code to follow us and explore the mysteries of chemistry together.